Research
musculoskeletal disease
The work in our group is dedicated to understanding mechanobiology in health and disease states. Our bodies rely on mechanical input to maintain functionally viable tissues. Bone, muscle and cartilage comprise the musculoskeletal system with mechanical load being central to function. Cells in these tissues are uniquely able to sense forces and turn them into biological signals through a process called mechanotransduction. We use intravital imaging techniques to observe cells within their tissues as they respond to mechanical forces in real time. Our objective is to use this knowledge to identify new targets for therapeutic intervention in musculoskeletal disease.
Check out Dr. Lewis’ Musculoskeletal Biology Workshop with the Orthopaedic Research Society Below! You can also listen to Dr. Lewis’ two minute podcast on Novel Approaches to Study Early Cell Changes in Musculoskeletal Disease at the Academic Minute here!
Bone Mechanobiology
-
biomechanics
Bone tissue plays the role of supporting ambulation and protecting our vital organs. It is a highly mineralized tissue organized into a mechanically relevant architecture.
-
mechanotransduction
Osteocytes are dendritic cells embedded throughout bone tissue within the lacuna-canalicular network. The chief role of osteocytes is to act as resident tissue mechanosensors, turning tissue-level strains into a diverse range of biological signals.

-
microcomputed tomography
We use micro-computed tomography (microCT) to investigate bone mass microarchitecture and duel x-ray absorptometry (DEXA) to determine mineral content

-
histomorphometry
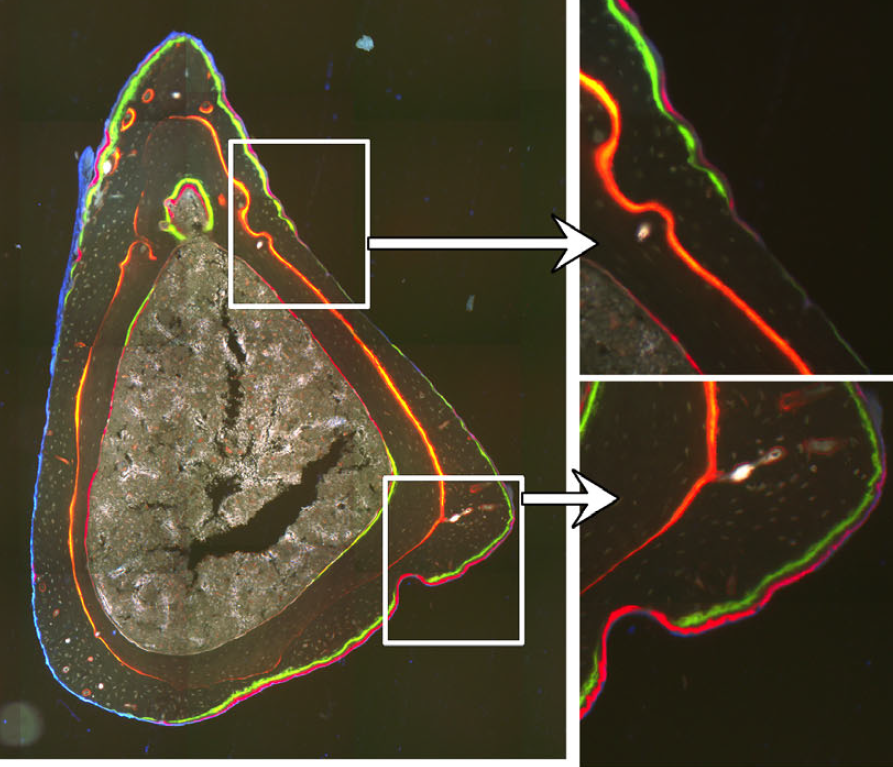
Osteoblasts and osteoclasts are bone building and eroding cells that shape, replace, and mechanically optimize bone tissue. We use histomorphometry to evaluate bone remodeling
In vivo Imaging
-
Metatarsal loading
We established a protocol for three-point bending device which is capable of delivering well-defined mechanical loads to metatarsal bones of living mice while simultaneously monitoring the intracellular Ca2+ responses of individual osteocytes by using a genetically encoded fluorescent Ca2+ indicator. Osteocyte responses are imaged by using multiphoton fluorescence microscopy.

-
Calcium Signaling
We generated a mouse model with an osteocyte-targeted genetically encoded Ca2+ indicator (GECI) to study Ca2+ signaling in osteocytes in their authentic in vivo environment. The mice have GCaMP to image fluorescent calcium signaling in osteocytes. A representative Ca2+ trace for a single osteocyte before (dashed line) and after the start of cyclical loading (solid line) at

-
Osteocyte mechanobiology
Little is known about how osteocytes in vivo acutely respond to tissue-level mechanical loading. We have demonstrated that osteocyte populations integrate mechanical signals by altering the number of responding cells and that this effect is dependent on loading frequency.




